¶ AOD-9604
Evidence: Failed late-stage clinical trials for obesity (lack of efficacy). Early-stage animal data for cartilage repair.
Safety: Well-tolerated in clinical trials, but placed on FDA Category 2 list (safety concerns/bulk drug substance restrictions) in 2023.
Action: Pioneers only. Banned by WADA. FDA restricted.
AOD-9604 (Anti-Obesity Drug 9604) is a synthetic peptide fragment comprising the C-terminal amino acids 177-191 of human growth hormone (hGH), with a tyrosine residue added to the N-terminus to enhance stability. It was originally developed by Metabolic Pharmaceuticals in Australia to mimic the fat-burning (lipolytic) effects of hGH without its anabolic (growth-promoting) or glycemic side effects.
While initially promising in animal models, AOD-9604 failed to demonstrate clinically meaningful weight loss in large-scale Phase 2b/3 human trials, leading to the discontinuation of its development as a pharmaceutical anti-obesity drug in 2007. It has since been marketed as a supplement and research compound, though recent FDA regulations have severely restricted its availability in the United States.
¶ Mechanism of Action
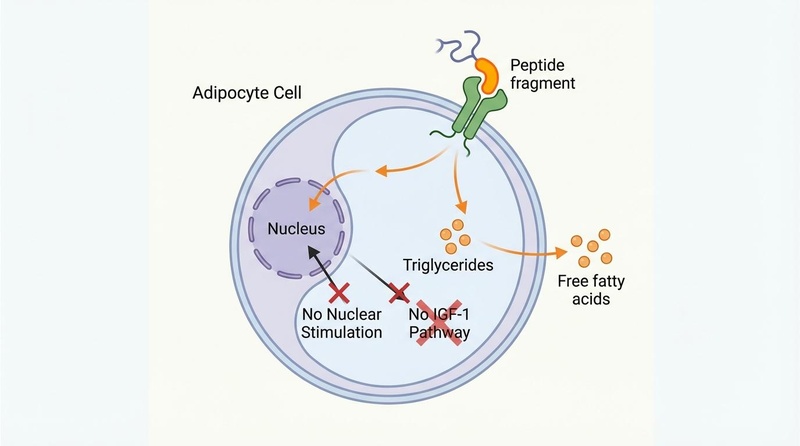
AOD-9604 represents the specific domain of the hGH molecule responsible for lipid metabolism.
¶ Targeted Lipolysis
Research suggests that the C-terminal region of hGH (residues 177-191) stimulates lipolysis (breakdown of fat) and inhibits lipogenesis (formation of new fat)[1]. AOD-9604 binds to receptors on adipocytes (fat cells) to trigger the release of stored fatty acids.
- No IGF-1 Release: Unlike full-length hGH, AOD-9604 does not bind to the hGH receptor with high affinity in the liver and does not stimulate the production of Insulin-Like Growth Factor 1 (IGF-1)[2].
- Metabolic Safety: Because it bypasses the IGF-1 axis, it avoids the insulin resistance and hyperglycemia often associated with chronic hGH therapy[2:1].
¶ Cartilage Regeneration (Secondary Mechanism)
Later research pivoted to musculoskeletal health, where AOD-9604 (sometimes referred to as LAT8881 in this context) showed potential to enhance cartilage repair.
- Studies in rabbits and rats suggested it could promote the regeneration of hyaline cartilage and improve collagen content in damaged joints, possibly by stimulating the proliferation of chondrocytes and proteoglycan synthesis[3][4].
¶ Clinical Evidence
¶ Obesity and Weight Loss (Failed)
The primary clinical program for AOD-9604 focused on obesity. Metabolic Pharmaceuticals conducted six human clinical trials involving over 900 subjects.
- The METAOD005 Trial: In a large Phase 2b study involving 300 obese participants, AOD-9604 was administered orally at doses of 1, 5, 10, 20, and 30 mg daily for 12 weeks.
- Results: The lowest dose (1 mg) showed a modest weight loss of ~2.6 kg vs. 0.8 kg for placebo (a difference of 1.8 kg). However, higher doses failed to produce significant weight loss, and the results were not considered dose-dependent or clinically robust enough to warrant Phase 3 investment[5].
- Outcome: The lack of a clear dose-response relationship and marginal efficacy led to the termination of the obesity program in 2007.
¶ Osteoarthritis
Following the failure in obesity, research shifted to osteoarthritis (OA).
- Animal Data: Demonstrated efficacy in collagenase-induced knee osteoarthritis models in rabbits, showing improved lameness scores and reduced cartilage degradation[3:1].
- Human Data: There is a lack of published, large-scale peer-reviewed human trials confirming efficacy for osteoarthritis, although it has been used off-label in integrative clinics.
¶ Safety and Regulatory Status
¶ Safety Profile in Trials
In the clinical trials conducted by Metabolic Pharmaceuticals, AOD-9604 was found to be safe and well-tolerated.
- Side Effects: Adverse events were indistinguishable from placebo. Common issues included mild headache or gastrointestinal upset, but no serious safety signals were reported[2:2].
- No Glucose Impairment: It did not induce glucose intolerance or insulin resistance, a key safety advantage over hGH.
¶ Regulatory Crackdown (FDA Category 2)
In September 2023, the U.S. FDA updated its policy on compounding bulk drug substances. AOD-9604 was placed in Category 2 of the 503A Bulks List.
- Meaning: The FDA determined there were "significant safety risks" or insufficient evidence of safety/efficacy to permit compounding. This effectively bans legitimate compounding pharmacies in the US from making AOD-9604 injections[6].
- WADA Ban: AOD-9604 is explicitly listed on the World Anti-Doping Agency (WADA) Prohibited List under S2 (Peptide Hormones, Growth Factors, Related Substances). It is banned in competitive sports at all times[7].
¶ Dosing
- Note: Due to regulatory restrictions, no FDA-approved dosing exists.
- Clinical Trial Dosing: The "effective" dose in the failed obesity trial was 1 mg orally per day.
- Subcutaneous: In "biohacking" communities, a common protocol is 300 mcg injected subcutaneously once daily, typically in the morning on an empty stomach.
- Cartilage Repair: Protocols for joint health often involve intra-articular injections (performed by a physician) or systemic subcutaneous use, though standard dosing is not established.
¶ References
Ng FM, et al. Metabolic studies of a synthetic lipolytic domain (AOD9604) of human growth hormone. Horm Res. 2000;53(6):274-8. ↩︎
Stier H, Vos E, Kenley D. Safety and Tolerability of the Hexadecapeptide AOD9604 in Humans. J Endocrinol Metab. 2013;3(1-2):7-15. ↩︎ ↩︎ ↩︎
Kwon DR, Park GY. Effect of Intra-articular Injection of AOD9604 with or without Hyaluronic Acid in Rabbit Osteoarthritis Model. Ann Clin Lab Sci. 2011;41(3):219-27. ↩︎ ↩︎
Kim KS, et al. The effects of AOD9604 on the regeneration of damaged cartilage. Journal of Orthopaedic Research. 2015. ↩︎
Metabolic Pharmaceuticals. Clinical Trial Results (METAOD005). Company Announcement. 2007. ↩︎
U.S. Food and Drug Administration. Interim Policy on Compounding Using Bulk Drug Substances Under Section 503A of the Federal Food, Drug, and Cosmetic Act. 2023. ↩︎
World Anti-Doping Agency. Prohibited List. S2. Peptide Hormones, Growth Factors, Related Substances, and Mimetics. ↩︎