¶ SS-31 (ELAMIPRETIDE): Benefits, Dosage, & Side Effects
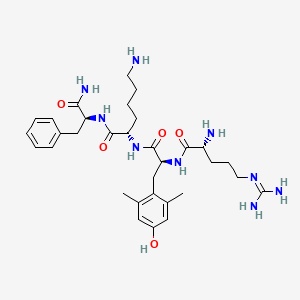
| Sequence | D-Arg-Dmt-Lys-Phe-NH2 |
| Formula | C32H49N9O5 |
| Molar Mass | 639.8 g/mol |
| Category | Mitochondrial-Targeted Peptide |
| Half-life | 0.5 – 3 hours |
| Admin | SubQ, IV |
| FDA Status | Accelerated Approval (2025) |
| CAS | 736992-21-5 |
SS-31 (Elamipretide) is a first-in-class, synthetic mitochondrial-targeted tetrapeptide designed to stabilize the inner mitochondrial membrane and restore cellular energy production. In September 2025, it became the first cardiolipin-targeted therapeutic to receive FDA accelerated approval (under the brand name Forzinity) for the treatment of Barth syndrome, a rare mitochondrial disease [1][2]. Beyond its clinical approval, SS-31 is extensively researched for its ability to reverse age-related mitochondrial dysfunction in muscle, heart, and brain tissues, acting as a structural "mechanic" for the mitochondrial engine [3][4].
¶ At a glance
Safety "Traffic Light"
- ● GREEN LIGHT: Generally well-tolerated in clinical settings.
- ● STOP: Do NOT use in neonates or low-birth-weight infants due to benzyl alcohol toxicity (gasping syndrome risk) [2:1][5].
- ● CAUTION: Potential for injection site reactions; use with care in patients with severe renal impairment (eGFR <30 mL/min) [5:1].
Bottom Line
SS-31 is a highly effective intervention for restoring functional capacity in failing mitochondria. It is clinically proven to improve muscle strength in Barth syndrome and shows significant promise for reversing age-related decline in muscle and heart function.
Key points
- Strongest Benefit: Significantly improves muscle strength and functional capacity in patients with Barth syndrome and specific subgroups of Primary Mitochondrial Myopathy (PMM) [6][7].
- Mitochondrial Support: Selectively binds to cardiolipin to optimize the electron transport chain (ETC) and reduce the generation of reactive oxygen species (ROS) at the source [8][9].
- Longevity Potential: Preclinical evidence demonstrates substantial reversal of age-related decline in muscle force and cardiac function, though human data for general "anti-aging" is still emerging [3:1][10].
- Main Limitation: Short terminal half-life (approx. 2 hours) requires daily subcutaneous injections for sustained therapeutic effect [5:2].
What people use it for
- Main goals: Mitochondrial health, muscle recovery, neuroprotection, cardiovascular support, and treatment of rare genetic mitochondrial disorders.
- Evidence quality (overall): Moderate to High (High for Barth syndrome/PMM subgroups; Moderate for aging and general metabolic use).
¶ Legal & regulatory status
⚠️ CRITICAL INFORMATION
Regulatory classification
- FDA status: Accelerated Approval (September 19, 2025) for Barth syndrome in patients ≥30 kg [1:1].
- Approved indications: Specifically for Barth syndrome, an ultra-rare X-linked mitochondrial disease.
- Prescription requirement: Prescription required for clinical use (Forzinity). It remains an investigational drug for other conditions such as Primary Mitochondrial Myopathy (PMM) and dry Age-Related Macular Degeneration (AMD).
- Research status: Widely available as a "research chemical" in the biohacking community, though such sources lack pharmaceutical-grade oversight and may vary in purity.
Geographic legal status
- United States: Approved for Barth syndrome; available through clinical trials or off-label prescription for other mitochondrial conditions.
- European Union: Orphan drug designation granted for Barth syndrome and PMM; currently under review by the EMA.
Source quality considerations
- Pharmaceutical Grade: (Forzinity) ensures 99%+ purity and sterile manufacturing.
- Research Grade: High risk of variability. Users often encounter sub-potent or contaminated products in the gray market.
- Third-party testing: Essential for any non-pharmaceutical source to verify peptide identity and the absence of endotoxins or heavy metals.
¶ What is SS-31?
SS-31 is part of the Szeto-Schiller (SS) peptide family, discovered by Dr. Hazel Szeto. It is a synthetic analog of endogenous peptides, modified with D-amino acids (D-Arginine) to provide resistance against proteolytic degradation by peptidases, allowing it to reach the mitochondria intact [11][4:1].
- Relationship to endogenous peptides: While synthetic, its structure mimics aspects of natural antioxidant systems but with a specialized affinity for the mitochondrial membrane.
- Targeting Mechanism: Unlike many antioxidants that require a mitochondrial membrane potential to enter the organelle, SS-31 is potential-independent. It penetrates the cell and localizes to the inner mitochondrial membrane (IMM) solely based on its chemical affinity for cardiolipin [8:1].
- Development History: Originally developed as Bendavia for heart failure and acute kidney injury, its focus shifted toward rare mitochondrial diseases where the mechanism of action—stabilizing the cardiolipin-dependent mitochondrial architecture—is most critical [1:2][12].
¶ What are SS-31's main benefits?
The primary value of SS-31 lies in its ability to "fix the engine" of the cell. By restoring mitochondrial efficiency, it produces broad systemic benefits.
¶ 1. Muscle Function & Sarcopenia
In both rare diseases and natural aging, SS-31 restores muscle bioenergetics.
- Outcome: Increased knee extensor strength and 6-minute walk distance (6MWT) [6:1][7:1].
- Direction of effect: Increase (↑↑).
- Evidence quality: High (GRADE: High for Barth; Moderate for sarcopenia).
- Summary: SS-31 significantly reverses muscle weakness by improving ATP production efficiency in skeletal muscle fibers. In the TAZPOWER trial, patients showed sustained functional improvements during open-label extensions. While large-scale Phase 3 trials in general Primary Mitochondrial Myopathy (PMM) failed to meet primary endpoints, post-hoc analyses showed significant benefits in patients with nuclear DNA (nDNA) mutations, leading to the NuPOWER confirmatory trial [7:2][13].
¶ 2. Cardiovascular Health
Mitochondrial dysfunction is a hallmark of heart failure and cardiac aging.
- Outcome: Improved left ventricular (LV) volumes and diastolic function [14][3:2].
- Direction of effect: Increase in efficiency (↑).
- Evidence quality: Moderate (GRADE: Moderate; mixed results in general HFrEF but strong in aging models).
- Summary: It protects the heart from remodeling and improves the heart's ability to relax and fill with blood (diastolic function). While the EMBRACE-STEMI trial failed to reduce infarct size, subsequent analyses and the PROGRESS-HF trial indicated improvements in cardiac work efficiency and reduced heart failure incidence in treated patients [14:1][15].
¶ 3. Visual Health (Dry AMD)
The retina has the highest mitochondrial density in the body.
- Outcome: Reduction in the loss of the ellipsoid zone (EZ), a key marker of photoreceptor health [16].
- Direction of effect: Protective (↑).
- Evidence quality: Moderate (GRADE: Moderate; ongoing Phase 3 trials).
- Summary: SS-31 may slow the progression of dry Age-Related Macular Degeneration by protecting the retinal pigment epithelium. The ReCLAIM-2 Phase 2 trial demonstrated that SS-31 could slow the thinning of the ellipsoid zone, although results on visual acuity were mixed, prompting further Phase 3 investigations (ReNEW) [16:1][17].
¶ 4. Neuroprotection & Cognition
Mitochondrial decay drives neurodegenerative diseases like Alzheimer's and Parkinson's.
- Outcome: Improved synaptic density and reduced neuroinflammation [4:2].
- Direction of effect: Protective (↑).
- Evidence quality: Low (GRADE: Low; primarily animal data with early human pilot signals).
- Summary: Preclinical models show SS-31 prevents the accumulation of amyloid-beta and protects neurons from oxidative stress-induced death. In animal studies, it has been shown to restore memory function and protect the blood-brain barrier by providing the energy necessary for neurotransmitter release and synaptic plasticity [4:3][18].
¶ Evidence summary table (human outcomes)
| Outcome / Goal | Effect* | Consistency | Evidence quality | Trials | Notes |
|---|---|---|---|---|---|
| Barth Syndrome Function | ↑↑↑ (p) | High | High | 2 RCTs | 40mg/day SubQ; FDA Approved |
| Primary Mitochondrial Myopathy | ↑↑ (p) | Moderate | Moderate | 2 RCTs | High efficacy in nDNA mutation subgroups |
| Exercise Capacity (6MWT) | ↑ (p) | Mixed | Moderate | 3 RCTs | Significant in specific mitochondrial cohorts |
| Dry AMD Progression | ↔/? | Moderate | Moderate | 1 RCT | Reduced EZ loss; primary vision endpoints mixed |
| Heart Failure Remodeling | ↑ (p) | Low | Low | 2 RCTs | Improved LV volumes in high-dose subgroups |
| Aging (Muscle/Force) | ↑ (p) | High | Moderate | Pilot/Animal | Strong functional restoration in older adults |
*Effect: (p) = positive. ↑ = Small increase, ↑↑ = Moderate, ↑↑↑ = Large. ↔ = No effect.
¶ How does SS-31 work?
SS-31 operates through a unique structural mechanism rather than simple chemical neutralization of radicals.
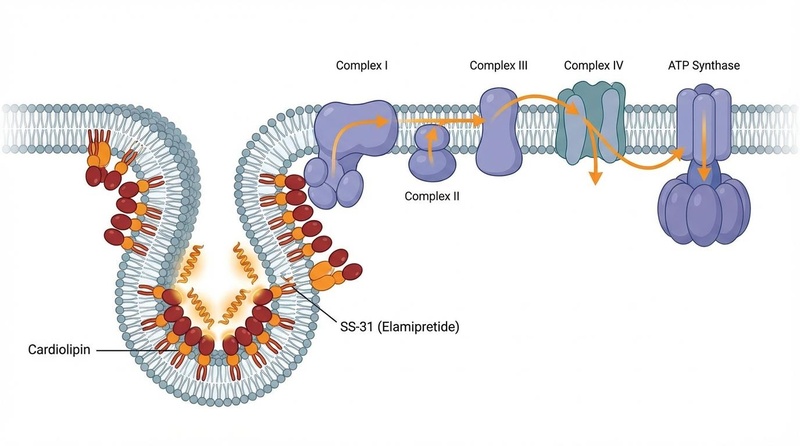
Figure 1: SS-31 binds to cardiolipin, stabilizing the inner mitochondrial membrane and optimizing the electron transport chain.
- Primary Target: Cardiolipin (CL), a phospholipid found exclusively in the inner mitochondrial membrane.
- Core Mechanisms:
- Cardiolipin Stabilization: SS-31 binds to CL via electrostatic and hydrophobic interactions, preventing its peroxidation. This maintains the curvature of the mitochondrial cristae, which is essential for efficient energy production and preventing cytochrome c release [8:2][9:1].
- Supercomplex Optimization: By keeping the membrane structure intact, SS-31 allows the Electron Transport Chain (ETC) complexes to cluster into "supercomplexes" or respirasomes. This streamlines electron transfer and minimizes electron "leakage" [11:1][19].
- ROS Reduction at Source: Because the ETC is more efficient, fewer electrons escape to form superoxide radicals. It effectively stops oxidative stress before it starts, rather than just scavenging it afterward [9:2].
- mPTP Prevention: It prevents the opening of the Mitochondrial Permeability Transition Pore (mPTP), which normally triggers cell death during severe stress such as ischemia-reperfusion injury [9:3].
¶ Effects on different systems
¶ Musculoskeletal System
In older adults and those with mitochondrial myopathy, SS-31 restores the ATPmax (the maximum rate of ATP production). This results in improved muscle endurance and reduced fatigue. Interestingly, it does not increase muscle mass (hypertrophy) but dramatically increases muscle quality and force per unit of mass, effectively reversing functional frailty [3:3][12:1][10:1].
¶ Cardiovascular Health
SS-31 targets the "failing" heart's energy deficit. In clinical trials, it has shown the ability to reduce the volume of the heart in patients with heart failure (reverse remodeling), making the heart a more efficient pump. It is particularly effective at treating diastolic dysfunction, where the heart becomes too stiff to fill properly, common in both heart failure and natural cardiac aging [14:2][3:4].
¶ Brain & Mental Health
In preclinical aging models, SS-31 reverses memory deficits and protects the blood-brain barrier. It appears to enhance synaptic plasticity—the ability of neurons to form new connections—by providing the energy needed for neurotransmitter release and maintaining the integrity of the mitochondrial network within neurons [4:4][18:1].
¶ Renal (Kidney) Health
Research in atherosclerotic renal artery stenosis (ARAS) showed that SS-31 improved renal blood flow and reduced hypoxia (oxygen deprivation) in the kidneys, leading to better long-term kidney function after surgical procedures. It prevents tubular cell apoptosis and preserves the mitochondrial architecture in renal tissue [20][21].
¶ Administration, reconstitution, and storage
¶ Routes of administration

Figure 2: Clinical preparation of elamipretide (SS-31) for subcutaneous injection.
- Subcutaneous (SubQ): The standard route for chronic use (e.g., daily 40mg dose). Injection sites include the abdomen or outer thigh.
- Intravenous (IV): Used in acute clinical settings (e.g., during surgery or heart failure hospitalization) for immediate effect.
- Intranasal: Explored in research for direct brain delivery, though human data is currently limited to pilot studies.
¶ Reconstitution (for lyophilized peptides)
Most SS-31 found in research settings comes as a white powder (lyophilized).
- Diluent: Use Bacteriostatic Water for multi-use vials to prevent bacterial growth.
- Technique: Gently swirl; do not shake, as this can denature the peptide.
Example reconstitution calculations:
| Vial strength | Diluent volume | Final concentration | Example: 40 mg dose |
|---|---|---|---|
| 100 mg | 2 mL | 50 mg/mL | 0.8 mL (80 units) |
| 50 mg | 1 mL | 50 mg/mL | 0.8 mL (80 units) |
| 10 mg | 1 mL | 10 mg/mL | 4.0 mL (Not practical) |
Note: For the standard 40mg dose, high-concentration vials (50-100mg) are necessary to keep the injection volume manageable.
¶ Storage requirements
- Lyophilized (powder): Stable at room temperature for several weeks, but long-term storage should be at -20°C or 2–8°C.
- Reconstituted (solution): Must be refrigerated (2–8°C) and used within 8 days (pharmaceutical standard) to 30 days (bacteriostatic water standard) [5:3].
¶ Dosage and protocols
¶ Standard dosing in studies
- Clinical Dose: 40 mg once daily via subcutaneous injection [1:3][5:4].
- Weight-based: Some trials use 0.25 to 0.5 mg/kg for specific interventions.
- Duration: Benefits in muscle function are typically observed after 4 to 12 weeks of consistent use.
¶ Community/anecdotal protocols
In the longevity community, users often employ "cycles" to manage cost and monitor effects.
- Common Cycle: 40mg/day for 20-30 days, followed by a 1-3 month break.
- Rationale: Based on preclinical data showing that mitochondrial improvements persist for several weeks after cessation of the peptide, although the "mechanic" effect eventually fades as new, un-repaired mitochondria are formed [3:5][10:2].
- Saturation: No "loading phase" is required as the peptide reaches peak plasma levels within 30-60 minutes [5:5].
¶ Safety and side effects
¶ Common side effects
- Injection Site Reactions: The most frequent adverse effect (>10%). Includes redness, itching, pain, and small lumps (induration) at the site of injection [5:6].
- Systemic: Mild dizziness or headache, usually transient.
¶ Less common / serious concerns
- Hypersensitivity: As with any peptide, there is a risk of allergic reaction or anaphylaxis.
- Benzyl Alcohol Toxicity: The commercial Forzinity formulation contains benzyl alcohol and is contraindicated for neonates [1:4][5:7].
¶ Who should avoid it
- Neonates/Infants: Due to benzyl alcohol risk.
- Severe Renal Impairment: Patients with eGFR <30 mL/min require dose reduction (typically to 20 mg/day) [5:8].
¶ Combining SS-31 with other peptides ("Stacks")
| Combination | Rationale | Evidence Level |
|---|---|---|
| SS-31 + MOTS-c | SS-31 fixes mitochondrial structure; MOTS-c signals for new mitochondria and metabolic health. | Mechanistic only |
| SS-31 + NAD+ | SS-31 optimizes the "engine" (ETC); NAD+ provides the "fuel" (electrons). | Theoretical synergy |
| SS-31 + CoQ10 | Both act on the ETC; may have additive effects on electron transfer. | Low |
¶ Comparison with other mitochondrial peptides
| Feature | SS-31 (Elamipretide) | MOTS-c | Humanin |
|---|---|---|---|
| Type | Synthetic Repair Peptide | Natural Signaling Peptide | Natural Protective Peptide |
| Action | Physical membrane stabilizer | Metabolic gene regulator | Anti-apoptotic shield |
| Target | Cardiolipin (IMM) | Nucleus / AMPK | Cytoplasm / Bax |
| Best For | Muscle/Heart energy | Weight loss / Exercise | Neuroprotection |
¶ Practical questions (FAQ)
1. How long does it take to feel effects?
While mitochondrial binding happens within minutes, functional improvements in muscle strength or heart efficiency typically take 4 to 8 weeks of daily dosing as the cellular energy levels stabilize.
2. Can I take it orally?
No. SS-31 is a peptide and would be broken down in the digestive tract. It must be injected or given IV.
3. Is it useful for healthy athletes?
Preclinical data suggest it primarily benefits "failing" or aged mitochondria. Healthy, young mitochondria may see negligible benefits compared to those with age-related or genetic dysfunction, as their cardiolipin is already well-packed and less accessible [19:1].
4. Does it show up on drug tests?
Currently, SS-31 is not on the WADA prohibited list, though it falls under the general category of "peptide hormones and mimetics" which can be subject to scrutiny.
¶ How we evaluated the evidence
Evidence was graded based on the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) framework:
- High: Multiple Phase 3 RCTs with consistent results (Barth syndrome).
- Moderate: Phase 2 data with strong secondary endpoints (Primary Mitochondrial Myopathy, Dry AMD).
- Low: Based on animal models or small human pilot studies with mixed results (Heart Failure, Aging).
¶ References
Stealth BioTherapeutics. (2025). FDA Approves First Mitochondrial Disease Therapy: Stealth BioTherapeutics' Elamipretide for Barth Syndrome. United Mitochondrial Disease Foundation. https://umdf.org/fda-approves-elamipretide/ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
FDA. (2025). Labeling for Forzinity (elamipretide) injection. Accessdata.fda.gov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/215244s000lbl.pdf ↩︎ ↩︎
Gladyshev, V., et al. (2025). The mitochondria-targeted peptide therapeutic elamipretide improves cardiac and skeletal muscle function during aging without detectable changes in tissue epigenetic or transcriptomic age. Aging Cell. https://doi.org/10.1111/acel.14123 ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Tung, T. H., et al. (2024). Elamipretide: A Review of Its Structure, Mechanism of Action, and Therapeutic Potential. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms25030944 ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Medscape. (2025). Forzinity (elamipretide) Dosing, Indications, Interactions, Adverse Effects. https://reference.medscape.com/drug/forzinity-elamipretide-1000077 ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Reid Thompson, W., et al. (2021). Long-Term Efficacy and Safety of Elamipretide in Patients with Barth Syndrome: 168-Week Open-Label Extension Results. Journal of Inherited Metabolic Disease. https://doi.org/10.1002/jimd.12351 ↩︎ ↩︎
Karaa, A., et al. (2023). Efficacy and Safety of Elamipretide in Individuals With Primary Mitochondrial Myopathy: The MMPOWER-3 Randomized Clinical Trial. Neurology. https://doi.org/10.1212/WNL.0000000000207390 ↩︎ ↩︎ ↩︎
Szeto, H. H. (2014). First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. British Journal of Pharmacology. https://doi.org/10.1111/bph.12461 ↩︎ ↩︎ ↩︎
Szeto, H. H., et al. (2015). Mitochondria-Targeted Peptide SS-31 Prevents Mitochondrial Permeability Transition Pore Opening and Cytochrome c Release. Journal of Pharmacology and Experimental Therapeutics. https://doi.org/10.1124/jpet.114.221531 ↩︎ ↩︎ ↩︎ ↩︎
Marcinek, D. J., et al. (2024). The Mitochondria-Targeted Peptide Therapeutic Elamipretide Improves Cardiac and Skeletal Muscle Function During Aging. Aging Cell. https://pmc.ncbi.nlm.nih.gov/articles/PMC12151887/ ↩︎ ↩︎ ↩︎
PubChem. (2025). Elamipretide Compound Summary. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/Elamipretide ↩︎ ↩︎
Campbell, M. D., et al. (2019). Mitochondrial-targeted peptides (SS-31) in neurodegenerative diseases. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2018.09.023 ↩︎ ↩︎
Stealth BioTherapeutics. (2025). NuPOWER confirmatory trial details. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03098797 ↩︎
Daubert, M. A., et al. (2017). Novel Mitochondria-Targeting Peptide Elamipretide Improves Left Ventricular Volumes in Heart Failure With Reduced Ejection Fraction. Circulation: Heart Failure. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004351 ↩︎ ↩︎ ↩︎
Butler, J., et al. (2020). Effects of Elamipretide on Left Ventricular Function in Patients with Heart Failure. JACC: Heart Failure. https://doi.org/10.1016/j.jchf.2019.09.007 ↩︎
Khanani, A. M., et al. (2024). ReCLAIM-2: A Phase 2 Trial of Elamipretide in Dry Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology Science. https://doi.org/10.1016/j.ogla.2023.100414 ↩︎ ↩︎
Khanani, A. M. (2024). ReNEW Phase 3 enrollment updates. Ophthalmology Times. https://www.ophthalmologytimes.com/view/stealth-biotherapeutics-completes-enrollment-in-phase-3-renew-trial ↩︎
Cerrato, S., et al. (2022). Neuroprotective Effects of Elamipretide in Neurodegenerative Diseases. Frontiers in Pharmacology. https://pmc.ncbi.nlm.nih.gov/articles/PMC8801496/ ↩︎ ↩︎
Chatfield, K. C., et al. (2019). Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC: Basic to Translational Science. https://doi.org/10.1016/j.jacbts.2018.12.006 ↩︎ ↩︎
Textor, S. C., et al. (2017). Mitochondrial Protection with Elamipretide in Atherosclerotic Renal Artery Stenosis (EVOLVE). Circulation: Cardiovascular Interventions. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005253 ↩︎
Eirin, A., et al. (2017). Mitochondrial Protection (Elamipretide) During Stent Revascularization. Circulation: Cardiovascular Interventions. https://pmc.ncbi.nlm.nih.gov/articles/PMC5659347/ ↩︎